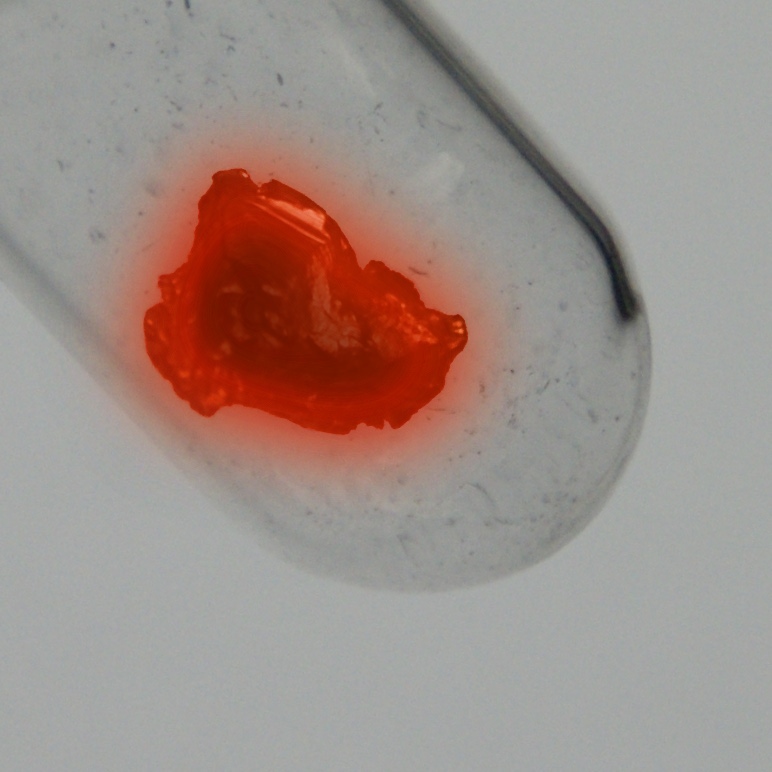
Protaktinij
91
Pa
Skupina
n/a
Perioda
7
Blok
f
Protoni
Elektroni
Neutroni
91
91
140
Opća svojstva
Atomski broj
91
Relativna atomska masa
231,03588
Maseni broj
231
Kategorija
Aktinoidi
Boja
Srebrna
Radioaktivan
Da
Od grčke riječi protos, prvi
Kristalna struktura
Prostorno centrirana tetragonska
Povijest
In 1900, William Crookes isolated protactinium as an intensely radioactive material from uranium
Protactinium was first identified in 1913 by Kasimir Fajans and Oswald Helmuth Göhring in Germany.
A more stable isotope of protactinium was discovered in 1917 by Otto Hahn and Lise Meitner at the Kaiser Wilhelm Institute in Berlin.
Protactinium was first identified in 1913 by Kasimir Fajans and Oswald Helmuth Göhring in Germany.
A more stable isotope of protactinium was discovered in 1917 by Otto Hahn and Lise Meitner at the Kaiser Wilhelm Institute in Berlin.
Elektrona po ljusci
2, 8, 18, 32, 20, 9, 2
Elektronska konfiguracija
[Rn] 5f2 6d1 7s2
Protaktinij je jedan od najrjeđih i najskupljih prirodnih elemenata
Fizikalna svojstva
Agregacijsko stanje
Čvrsto
Gustoća
15,37 g/cm3
Talište
1841,15 K | 1568 °C | 2854,4 °F
Vrelište
4300,15 K | 4027 °C | 7280,6 °F
Toplina taljenja
15 kJ/mol
Toplina isparavanja
470 kJ/mol
Specifični toplinski kapacitet
- J/g·K
Zastupljenost u Zemljinoj kori
9,9×10-13%
Zastupljenost u svemiru
n/a
CAS broj
7440-13-3
PubChem CID broj
n/a
Atomska svojstva
Atomski radijus
163 pm
Kovalentni radijus
200 pm
Elektronegativnost
1,5 (Paulingova ljestvica)
Potencijal ionizacije
5,89 eV
Atomski volumen
15,0 cm3/mol
Toplinska vodljivost
0,47 W/cm·K
Stanja oksidacije
3, 4, 5
Primjene
Owing to its scarcity, high radioactivity and high toxicity, there are currently no uses for protactinium outside of scientific research.
With the advent of highly sensitive mass spectrometers, an application of 231Pa as a tracer in geology and paleoceanography has become possible.
Protactinium-231 combined with the thorium-230 can be used to date marine sediments.
With the advent of highly sensitive mass spectrometers, an application of 231Pa as a tracer in geology and paleoceanography has become possible.
Protactinium-231 combined with the thorium-230 can be used to date marine sediments.
Protactinium is toxic and highly radioactive
Izotopi
Stabilni izotopi
-Nestabilni izotopi
212Pa, 213Pa, 214Pa, 215Pa, 216Pa, 217Pa, 218Pa, 219Pa, 220Pa, 221Pa, 222Pa, 223Pa, 224Pa, 225Pa, 226Pa, 227Pa, 228Pa, 229Pa, 230Pa, 231Pa, 232Pa, 233Pa, 234Pa, 235Pa, 236Pa, 237Pa, 238Pa, 239Pa, 240Pa