Radij
88
Ra
Skupina
2
Perioda
7
Blok
s
Protoni
Elektroni
Neutroni
88
88
138
Opća svojstva
Atomski broj
88
Relativna atomska masa
[226]
Maseni broj
226
Kategorija
Zemnoalkalijski metali
Boja
Srebrna
Radioaktivan
Da
Od latinske riječi radius, zraka
Kristalna struktura
Kubna prostorno centrirana
Povijest
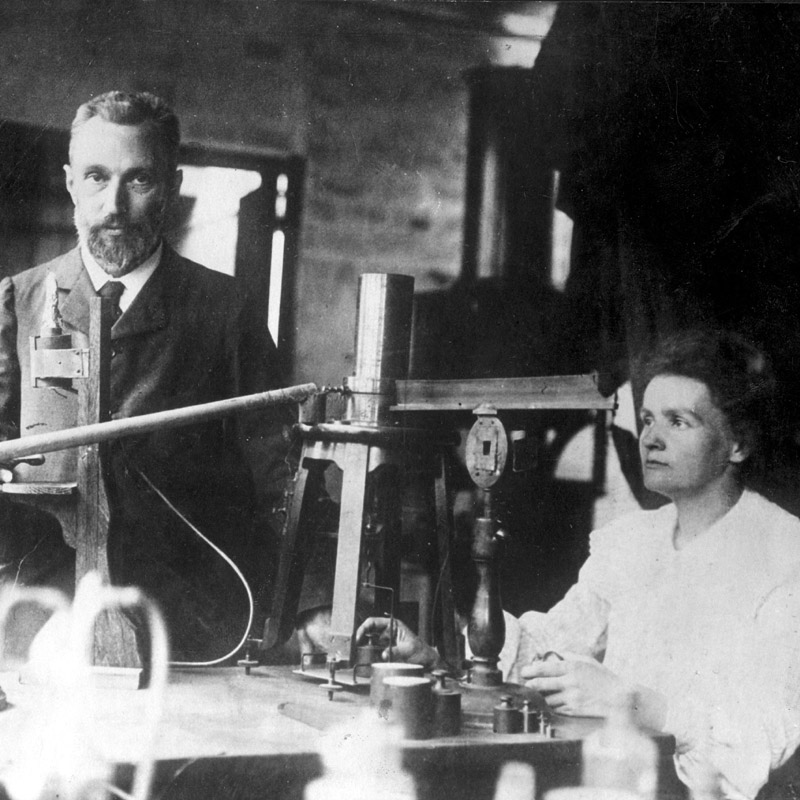
Radium was discovered by Marie Curie and Pierre Curie in 1898.
They extracted the radium compound from a uraninite sample.
Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne in 1910 through the electrolysis of radium chloride by using a mercury cathode and distilling in an atmosphere of hydrogen gas.
They extracted the radium compound from a uraninite sample.
Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne in 1910 through the electrolysis of radium chloride by using a mercury cathode and distilling in an atmosphere of hydrogen gas.
Elektrona po ljusci
2, 8, 18, 32, 18, 8, 2
Elektronska konfiguracija
[Rn] 7s2
Radij plamenu daje karmin crvenu boju
Fizikalna svojstva
Agregacijsko stanje
Čvrsto
Gustoća
5,5 g/cm3
Talište
973,15 K | 700 °C | 1292 °F
Vrelište
2010,15 K | 1737 °C | 3158,6 °F
Toplina taljenja
8 kJ/mol
Toplina isparavanja
125 kJ/mol
Specifični toplinski kapacitet
- J/g·K
Zastupljenost u Zemljinoj kori
9,9×10-12%
Zastupljenost u svemiru
n/a
CAS broj
7440-14-4
PubChem CID broj
6328144
Atomska svojstva
Atomski radijus
-
Kovalentni radijus
221 pm
Elektronegativnost
0,9 (Paulingova ljestvica)
Potencijal ionizacije
5,2784 eV
Atomski volumen
45,20 cm3/mol
Toplinska vodljivost
0,186 W/cm·K
Stanja oksidacije
2
Primjene
Radium was formerly used in self-luminous paints for watches, nuclear panels, aircraft switches, clocks, and instrument dials.
Radium chloride was used in medicine to produce radon gas which in turn was used as a cancer treatment.
The isotope 223Ra is currently under investigation for use in medicine as a cancer treatment of bone metastasis.
Radium chloride was used in medicine to produce radon gas which in turn was used as a cancer treatment.
The isotope 223Ra is currently under investigation for use in medicine as a cancer treatment of bone metastasis.
Radium is highly radioactive and carcinogenic
Izotopi
Stabilni izotopi
-Nestabilni izotopi
202Ra, 203Ra, 204Ra, 205Ra, 206Ra, 207Ra, 208Ra, 209Ra, 210Ra, 211Ra, 212Ra, 213Ra, 214Ra, 215Ra, 216Ra, 217Ra, 218Ra, 219Ra, 220Ra, 221Ra, 222Ra, 223Ra, 224Ra, 225Ra, 226Ra, 227Ra, 228Ra, 229Ra, 230Ra, 231Ra, 232Ra, 233Ra, 234Ra